BS ISO 17584 pdf download
BS ISO 17584 pdf download Refrigerant properties
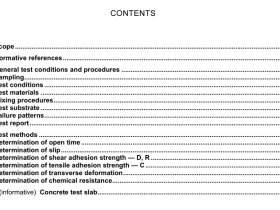
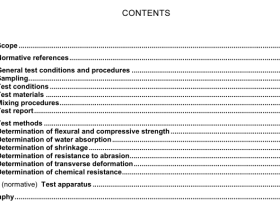
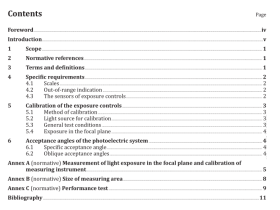
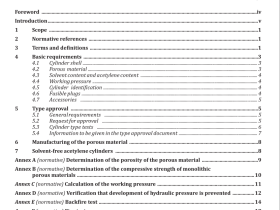
Scope
This nternational Standard specifies thermophysical properties of several commonly used refrigerants andrefrigerant blends.
This lnternational Standard is applicable to the refrigerants R12, R22, R32, R123, R125, R134a R143a.R152a, R717 (ammonia), and R744 (carbon dioxide) and to the refrigerant blends R404A, R407C, R410A.and R507A. The following properties are included: density, pressure, internal energy, enthalpy, entropy, heatcapacity at constant pressure, heat capacity at constant volume, speed of sound, and the Joule-Thomsoncoefficient,in both single-phase states and along the liquid-vapour saturation boundary. The numericadesignation of these refrigerants is that defined in ISO 817
2Normative references
The following referenced documents are indispensable for the application of this document. For datedreferences, only the edition cited applies. For undated references, the latest edition of the referenceddocument(including any amendments)applies
ISO 817,Refrigerants – Designation system
3 Terms and definitions
For the purposes of this document, the following terms and definitions apply.
31
algorithmprocedure for the computation of refrigerant properties
NOTEAn algorithm is most often a computer program. An algorithm may also consist of one or more single-propertycorrelations as allowed under 4.4.
3.2
blend
mixture of two or more chemical compounds
3.3
critical point
state at which the properties of the saturated liquid and those of the saturated vapour become equal
NOTESeparate liquid and vapour phases do not exist above the critical point temperature for a pure fluid. This ismore completely referred to as the “gas-liquid critical point” as other “critical points” can be defined.
3.4
equation of statemathematical equation that is acomplete and thermodynamically consistent representation of thethermodynamic properties of a fluid
NOTEAn equation of state most commonly expresses pressure or Helmholtz energy as a function of temperaturedensity, and (for a blend) composition, Other thermodynamic properties are obtained through integration and/ordifferentiation of the equation of state.
3.5 fluid
refrigerant
substance, present in liquid and/or gaseous states, used for heat transfer in a refrigerating system
NOTEThe fluid absorbs heat at a low temperature and low pressure, then releases the heat at a higher temperatureand a higher pressure, usually through a change of state.
3.6
liquid-vapour saturationstate at which liquid and vapour phases of a fluid are in thermodynamic equilibrium with each other at acommon temperature and pressure
NOTESuch states exist from the triple point to the critical point
3.7
transport properties
viscosity, thermal conductivity, and diffusion coefficient
3.8thermodynamic propertiesdensity, pressure, fugacity, internal energy, enthalpy, entropy, Gibbs and Helmholtz energies, heat capacitiesspeed of sound, and the Joule-Thomson coeficient, in both sinale-phase states and alona the liquid-vapoursaturation boundary
3.9
thermophysical propertiesall of the thermodynamic, transport, and other miscellaneous properties
3.10
triple point
state at which solid, liquid, and vapour phases of a substance are in thermodynamic equilibrium