ISO 1656 pdf download
ISO 1656 pdf download Rubber, raw natural, and rubber latex, natural — Determination of nitrogen content
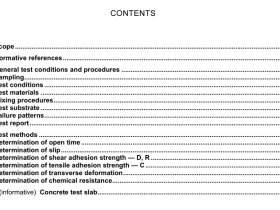
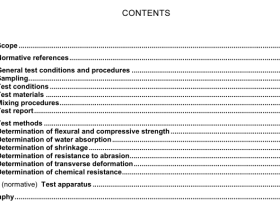
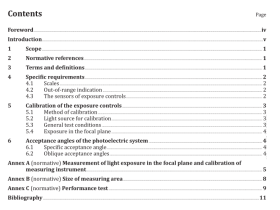
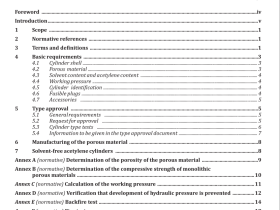
1 Scope
This International Standard specifies a macro-method and a semi-micro method for the determinationof nitrogen in raw natural rubber and in natural rubber latex using variants of the Kjeldahl process.
NOTEThe determination of nitrogen in natural rubber is usually carried out in order to arrive at an estimateof the protein content.Minor amounts of non-proteinous nitrogen containing constituents are also presentHowever, in the dry solids prepared from natural rubber latex these materials can make a substantial contributionto the total nitrogen content.
2 Normative references
The following documents, in whole or in part, are normatively referenced in this document and areindispensable for its application, For dated references, only the edition cited applies. For undatedreferences, the latest edition of the referenced document (including any amendments) applies.
ISO 123, Rubber latex – Sampling
ISO 124, Latex, rubber – Determination oftotal solids contentISO 1795, Rubber, raw natural and raw synthetic – Sampling and further preparative proceduresISO/TR 9272, Rubber and rubber products — Determination of precision for test method standards
3 Principle
A known mass of the sample is digested with a mixture of sulfuric acid, potassium sulfate and catalvticamounts of copper sulfate and selenium or sodium selenate, thereby converting nitrogen compoundsinto ammonium hydrogen sulfate from which the ammonia is distilled after making the mixture alkaline
The distilled ammonia is absorbed either in standard volumetric sulfuric acid solution followed bytitration of the excess acid with a standard volumetric base solution or in boric acid solution followedv titration with standard volumetric acid solution as boric acid is a weak acid. it does not affect theindicator used for this titration).
4Macro-method
4.1Reagents
During the analysis, unless otherwise stated, use only reagents of recognized analytical grade and onlydistilled water or water of equivalent purity.
4.1.1 Catalyst mixture or catalyst solution
CAUTION — When working with selenium, avoid breathing vapours and/or contact with skin or clothing. Work only with adequate ventilation.
4.1.1.1 Catalyst mixture
Prepare a finely divided intimate mixture of the following:
— 30 parts by mass of anhydrous potassium sulfate (K 2 SO 4 );
— four parts by mass of copper sulfate pentahydrate (CuSO 4 ·5H 2 O);
— one part by mass of selenium powder or two parts by mass of sodium selenate decahydrate (Na 2 SeO 4 ·10H 2 O).
4.1.1.2 Catalyst solution
Dissolve, with heating, the following:
— 110 g of anhydrous potassium sulfate;
— 14,7 g of copper sulfate pentahydrate;
— 3,7 g of selenium or 7,49 g of sodium selenate, in 600 cm 3 of sulfuric acid (4.1.2).
4.1.2 Sulfuric acid, ρ = 1,84 g/cm 3 .
4.1.3 Sulfuric acid standard volumetric solution, c(H 2 SO 4 ) = 0,05 mol/dm 3 .
4.1.4 Sodium hydroxide standard volumetric solution, c(NaOH) = 0,1 mol/dm 3 .
4.1.5 Sodium hydroxide solution, c(NaOH) approximately 10 mol/dm 3 .
Dissolve 400 g of solid sodium hydroxide in 600 cm 3 of water and dilute to 1 000 cm 3 .
4.1.6 Boric acid solution, c(H 3 BO 3 ) approximately 0,17 mol/dm 3 .
Dissolve 10,5 g of solid boric acid in water, warming if necessary, and dilute to 1 000 cm 3 then cool the solution to room temperature.
4.1.7 Mixed indicator solution.
Dissolve 0,1 g of methyl red and 0,05 g of methylene blue in 100 cm 3 of at least 95 % (V/V) ethanol.
This indicator might deteriorate during storage and shall therefore be freshly prepared.
4.2 Apparatus
Ordinary laboratory apparatus and Kjeldahl apparatus, with a digestion flask of capacity 800 cm 3